Inorganic synthesis
Find out about the work being done by this research group, which looks into synthesising inorganic compounds.
Synthetic {Inorganic} Chemistry group—S{I}C@Vic
Synthetic chemistry is the keystone of chemical research. In order to study something, you first need to make it. Even research in quantum chemical calculations can benefit from verification through experimentally derived data.
We do a lot of ‘inorganic chemistry’ research in our labs, but we also understand that the boundaries between inorganic/organic chemistry and materials science are not as distinct as they used to be.
Our interests lie in the synthesis and characterisation of the complex systems involving research in multiple disciplines and areas. Our projects span many areas involving the synthesis of inorganic and organic compounds as well as materials chemistry.

Academic staff
The Synthetic {Inorganic} Chemistry Group at Te Herenga Waka—Victoria University of Wellington is led by two academics who run independent research programs, but who share some common interests: Martyn Coles and Robin Fulton. Both lead researchers have extensive experience in laboratories around the world and are well established in their fields, with over 150 publications in the chemistry literature.
Prof John Spencer is conducting related research in organometallic chemistry at this university.
Professor of Chemistry
Associate Dean, Learning and Teaching
Wellington Faculty of Science
Techniques
Many of the compounds that are synthesised in our laboratories require special conditions to prepare and study. For example, most of the metal complexes that we make react with oxygen and moisture from the air (sometimes by spontaneously bursting into flames). We protect them from this by using a nitrogen filled glove-box and / or double manifold vacuum lines (or Schlenk lines).
Using these techniques we are able to safely handle and manipulate these reactive chemicals and use them extensively in our research projects.
In order to definitively prove the chemical composition of the new compounds that we make in our lab, we use a combination of analytical techniques. Most of the molecular systems we research contain NMR active nuclei and so we are able to use multi-nuclear experiments to examine the structures.
In addition to routine 1H and 13C NMR spectra that tell us about the ligands we have attached to the metal, where appropriate we also use experiments with 11B, 19F, 27Al, 29Si, 31P, 77Se, 119Sn, 195Pt, and so on that help to determine the structure of compounds in solution.
We also use variable temperature NMR studies to examine any fluxional behavior and obtain kinetic data, and frequently monitor reactions using this technique to observe reactions as they progress.
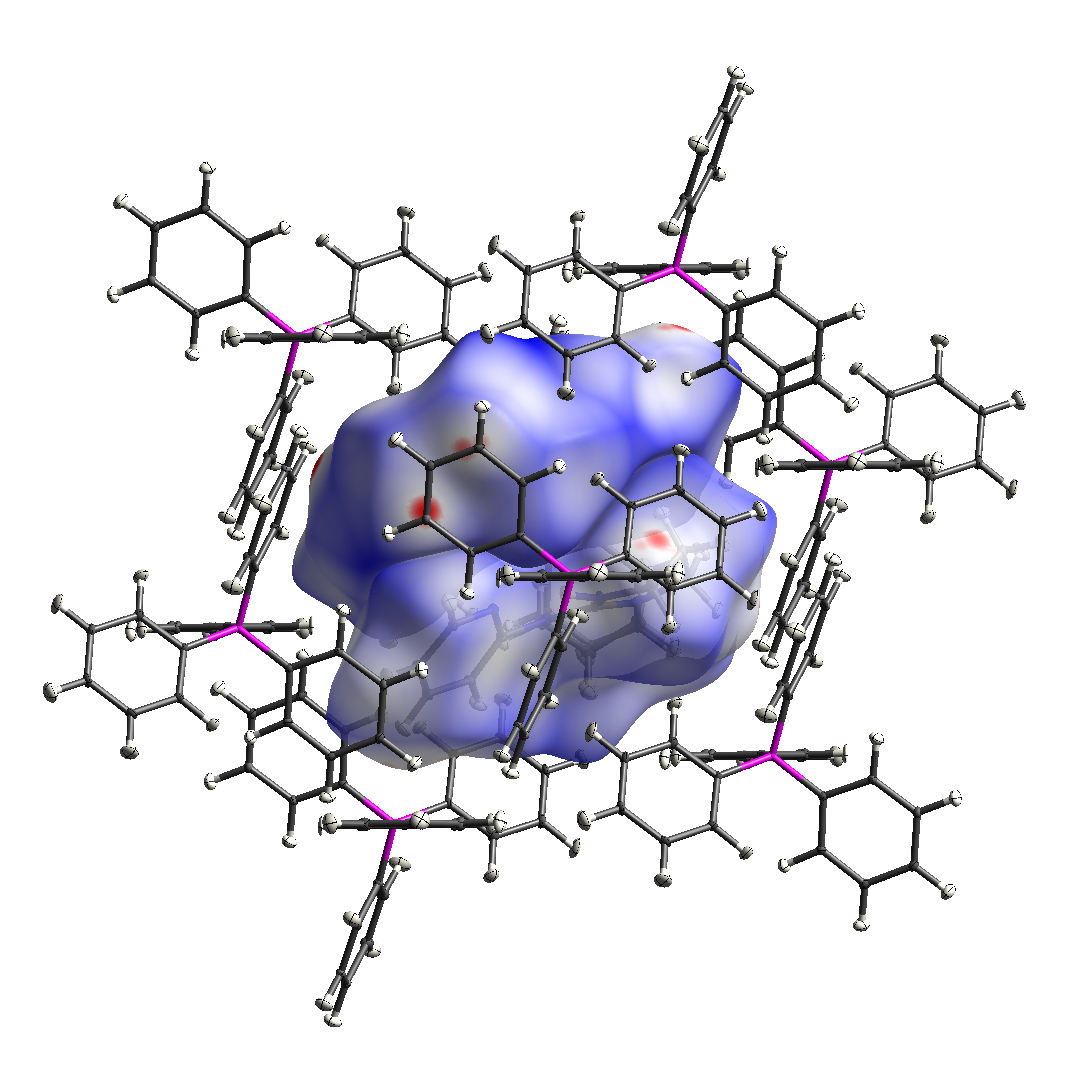
Perhaps the most powerful technique for determining the molecular structure of a compound is X-ray diffraction. Using our Aglient Dualsource SuperNova X-ray diffractometer, we can obtain accurate models of the atomic connectivity and crystal structure for complexes that form suitable crystalline phases.
The S{I}C group uses this instrument routinely. We have obtained structural information on compounds incorporating a range of metal elements, including Li, Mg, Al, K, Ga, Ge, In, Sn, Sb, Pb, Bi, and Sm.


