When I heard a few weeks ago that the Nobel Prize in Chemistry was awarded to the pioneers of lithium-ion battery research, I wondered: how different would my day-to-day life be without these rechargeable batteries?
For one, my morning commute would be boring without my podcasts. Rather than gazing out the window on the top floor of an electric double-decker bus, I'd be stuck in traffic in the back seat of a gas-guzzling Uber.
I'm sure I could get by just in this alternate reality, but the world's population living in poverty cannot. I was born and raised in India, the fourth largest energy consumer in the world.
Energy poverty disproportionately affects more than 400 million people, limiting their access to food and water, education and employment, and negatively impacting their health and hygiene. Rechargeable batteries will help ensure that people have access to the energy they need.
The issue with lithium-ion batteries is while they appear to be the leading technology to help mitigate the effects of climate change, they are not as sustainable as you may think. The most common metals in these batteries—lithium, nickel, cobalt—are not earth-abundant.
These materials are expensive to extract and their toxicity damages surrounding ecosystems. Because lithium-ion batteries are made with complex chemicals (electrolytes) that are hard to separate from each other, recycling them is an expensive and timely process.
As we continue to buy more lithium-ion batteries that finish their life-cycle in landfills, these elements will become increasingly rare and expensive. One day, there will be no lithium and cobalt left for us to use.
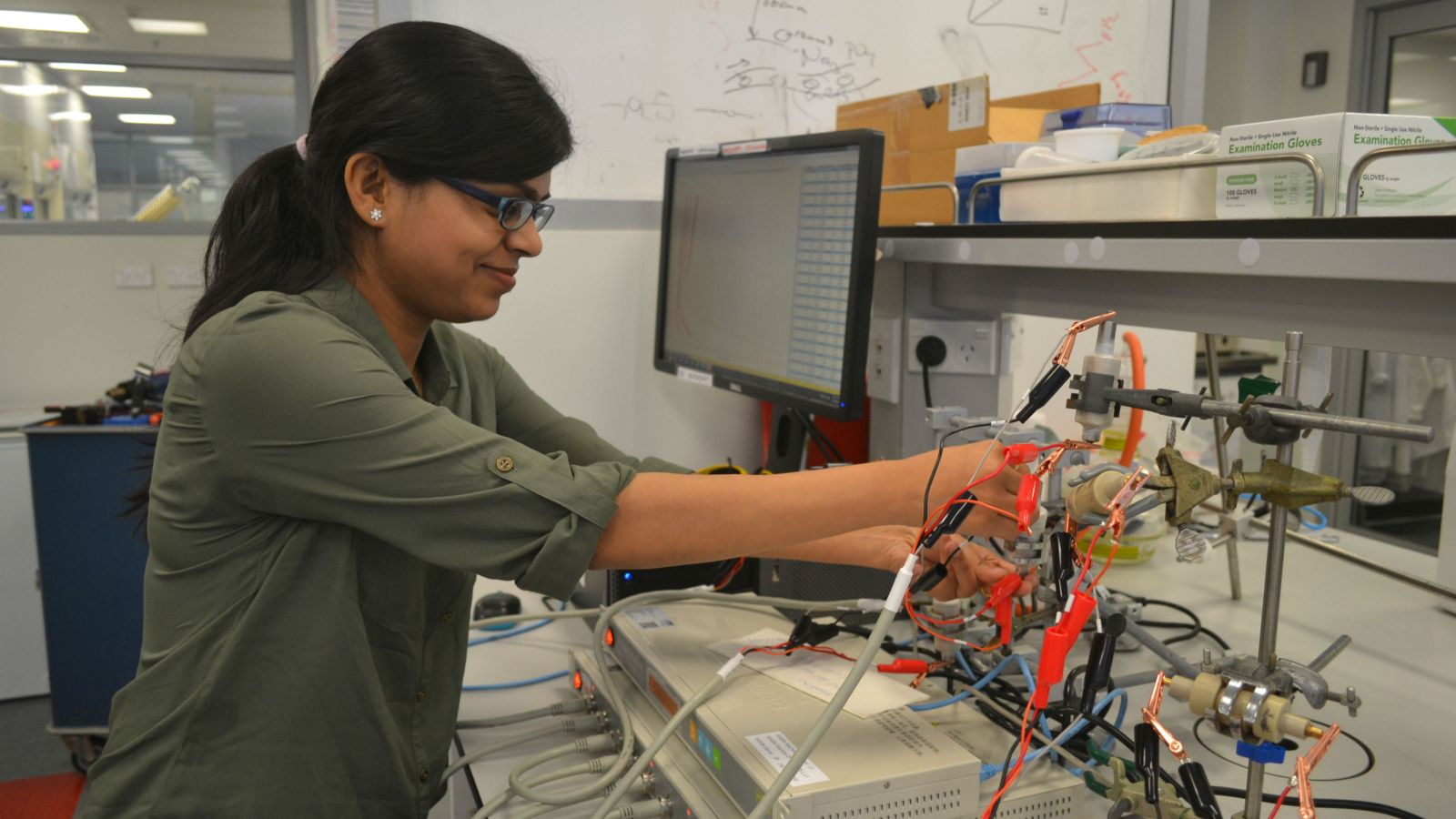
This is why I moved to New Zealand for my PhD, where I'm part of the MacDiarmid Institute and based at Victoria University of Wellington. I set off on a mission to find safe, cheap, and earth-abundant materials for sustainable batteries that will help us reduce our carbon footprint.
Batteries are made up of three parts: an anode, cathode, as well as an electrolyte solution to transfer charges between the two. My research is focused on the second part of the battery equation; the cathode.
The search for an alternative cathode material is challenging because this material must have certain features to allow reactions to occur within the cell.
Graphite (abundantly found in the Earth's crust) is a promising anode material in lithium-ion batteries and has been used extensively in aluminium-ion batteries too. It has good conductivity and a layered structure that allows easy movement of ions across the electrodes to charge the cell.
And recently researchers have discovered that aluminium can be used as an anode material in an alternative battery technology. Aluminium has a high energy storage capacity and is the third most abundant element in the Earth's crust.
My supervisors—former MacDiarmid Institute Principal Investigator Professor Thomas Nann (now at the University of Newcastle, Australia) and Professor Jim Johnston (Victoria University of Wellington)—believe in achieving new heights when it comes to research.
We knew there must be a material with a higher capacity and voltage than anything that had been published to date. Therefore, I built my own aluminium-ion batteries, using upwards of 60 different cathode materials, and found a material that outperforms both graphite and cathodes of lithium-ion batteries.
Battery research is interesting in its own way. A simple process of galvanostatic (constant current) charge and discharge gives me the most important information I need. It tells me whether the material will last or not after all these cycles, maintaining a high energy density at the same time.
Building batteries with earth-abundant materials is important. However, what is also important is for how much "longer" they can power our appliances (high energy density).
If we want to continue the transition away from fossil fuels to renewable sources of energy, we need powerful and efficient batteries. Renewable energy is intermittent; when the wind gusts die down and the sun disappears behind the clouds for too long, batteries will provide us with stored energy on demand.
It is likely we will not mass-produce these batteries here in New Zealand, but we can hold IP and benefit economically. We can also take pride in helping those in poverty live more secure, healthy and fulfilling lives.
Knowing that the work I'm doing here in Aotearoa may one day help lift millions of people back home out of poverty is what drives my passion to keep improving my research.
I always enjoy the opportunity to talk about my research, especially with younger university students, I see how engaged and interested they are in my work.
It leaves me feeling optimistic for the future, knowing that this next generation is motivated and willing to adapt to new technologies that will help keep their future green.
This story was originally published on Stuff.
Learn more about the MacDiarmid Institute on their website.
